Preparation Of Cholesterol Reference Standard
Abstract
Cholesterol is one of the principal sterols synthesized by all animals.Besides its importance for cell membranes, it also serves as a precurs or for the biosynthesis of steroid hormones, bile acid,and vitamin D. Cholesterol level in human body strongly influents the health, therefore, cholesterol level testing is now a routine work in clinical examinations. Commercial cholesterol is normally obtained from nature sources. The industrial purification of cholesterolis usually done using recrystallization in methanol, ethanoland acetic acid. However, the purity of the product purified by these methods is difficult to achieve 99% and higher, so that it is not able to meet the requirements of analytical standard material.The commercial cholesterol is usually contaminated by cholestan-3-ol, cholest-7-en-3-ol, and cholesta-5, 7-dien-3-oI, those are not able to be removed by recrystallization. This work reports apractical and large scale chemical purification through bromination and debromination process for preparation of higher purity cholesterol.
Results and Discussion
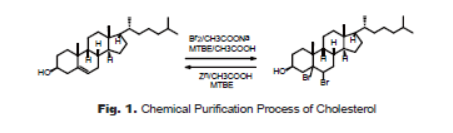
Chemical purification is a powerful method for many compounds that can’t be separated by normal purification techniques. The principal of chemical purification is to transform one of the compositions in the mixture to another more structural different compound through chemical reaction, then to achieve the separation of the two moieties. Cholesterol on the market is usually contains impurities, such as cholestan-3-ol, cholest-7-en-3-ol, and cholesta-5,7-dien-3-oI. These impurities can’t be removed by recrystallization.Bases on the different reactivity towards bromine, addition reactions of the C=C double bond in cholesterol (3β-cholest-5-en-3-ol) and impurities of 3β-cholest-7-en-3-ol and 3β-cholesta-5,7-dien-3-oI with bromine form brominated compounds(Fig. 1). Among them, the 5,6-dibromocholesterol is precipitated from the tert-butyl methyl ether solution, yet the brominated impurities, the oxidation products, 7-ketocholesterol and cholestane-3,25-diol, and other unreacted impurities remain in the solution because of the solubility differences. Filtration under vacuum achieves the separation of the product precipitated and impurities in the solution. In the subsequent reaction , the dibrimocholesterol was treated with zinc dust in acetic acid to regenerate the cholesterol by reductive debromination. Removal of inorganicsolid by filtration, the cholesterol solution was partially evaporated to afford cholesterol as white crystalline. To optimizethe reaction conditions, different solvents and reactant ratio are investigated. The best result is achieved in the first step of reactionat ratio of cholesterol : bromine = 1 : 1.1. tert-Butyl methylether is a better solvent than diethyl ether in large scale process.The second reaction is optimized at ratio of dibromochlesterol :zinc = 1 : 1.5, and using methanol as crystallization solvent. The process gives 80% yield in kilogram scale.
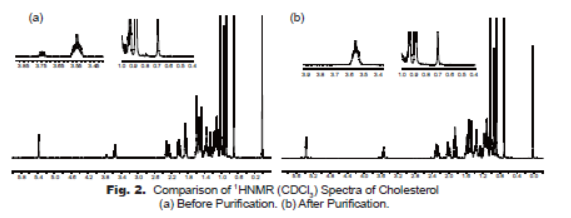
The product has been characterized by 1HNMR and HPLC. The 1HNMR spectrum (Fig. 2) is characteristic to the standard 1HNMR spectrum. As shown in the 1HNMR spectra, the impurities at 3.75 ppm, 0.75-0.9 ppm, 0.5-0.7 ppm before purification (Fig. 2-a) are almost completely removed through the two-step chemical purification process (Fig. 2-b).
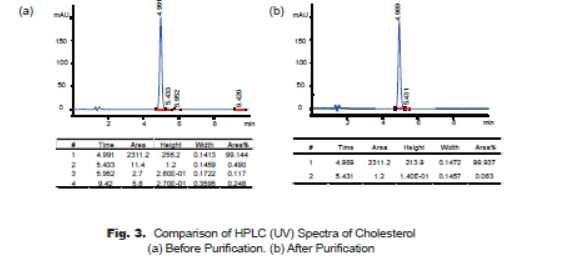
Because of the weak UV absorption property of cholesterol structure, the HPLC spectra are taken at 220nm, which looks like the better wavelength to reflect the purity of the product. HPLC spectra demonstrate that the major impurity at 5.43min (cholest-7-en-3-ol) is reduced from 0.49% to 0.06%, and the impurity at 5.95min is not observed after purification (Fig. 3-a, Fig. 3-b), the purity of cholesterol product is improved significantly to 99.9%.
Conclusion
We have reported a practical, large scale and high yield chemical purification method of cholesterol. The product is characterized by 1HNMR and HPLC , and the purity is up to 99.9%, suitable for using as reference standard in analysis of cholesterol level.
References
1. J AOAC Int, 2012, 95(2), 472-488. Thu T.N. Dinh, et. al. Determination of Total Cholesterol in Meat and Poultry by Gas Chromatography: Single-Laboratory Validation
2. Clinical Chem. 1963, 9(2), 123-134. N. Radin, et. al. Standard of purity for Cholesterol
3. J. Lipid Res, 1965, 6, 461-465. J. H. Williams, et. al. Purity of cholesterol to be used as a primary standard
4. AOAC Official Method 994.10, Cholesterol in Foods
5. Am J Anal Chem, 2012, 3, 306-311. G. L. Stroher, et. al. Comparative Analysis and Validation Methodologies of GC and HPLC for Analysis of Cholesterol in Meat Products.




